View the above photo record (by Gert Bensch) in OdonataMAP here.
Find the Sable Cruiser in the FBIS database (Freshwater Biodiversity Information System) here.
Family Macromiidae
Phyllomacromia monoceros – SABLE CRUISER
Identification
Large size
Length reaches 62mm; Wingspan attains 84mm.
Most similar to the two other Phyllomacromia species in the region, the Darting Cruiser (Phyllomacromia pictus) and the Two-banded Cruiser (Phyllomacromia contumax).
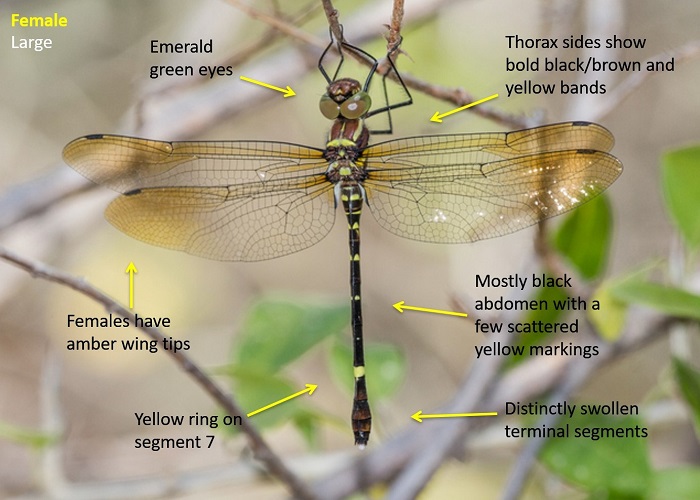
The Sable Cruiser can be identified by its predominantly black abdomen that shows only a few scattered yellow markings. There is a larger yellow ring on segment 7. The sides of the thorax show bold yellow and black bands. This is similar to that of Phyllomacromia pictus, but differs from the plain black thorax of Phyllomacromia contumax.
Males have a diagnostic vertical spine on the tenth abdominal segment.
Click here for more details on identification of the Sable Cruiser.
Nwanedi Nature Reserve, Limpopo
Photo by John Wilkinson
Habitat
Associated with forested habitats where it frequents rivers and streams. Often found hunting in nearby clearings or over wetlands at the forest edge.
Behaviour
Most often encountered in flight. Patrols back and forth along a regular route. The flight is fast and powerful. Hangs from a perch when at rest.
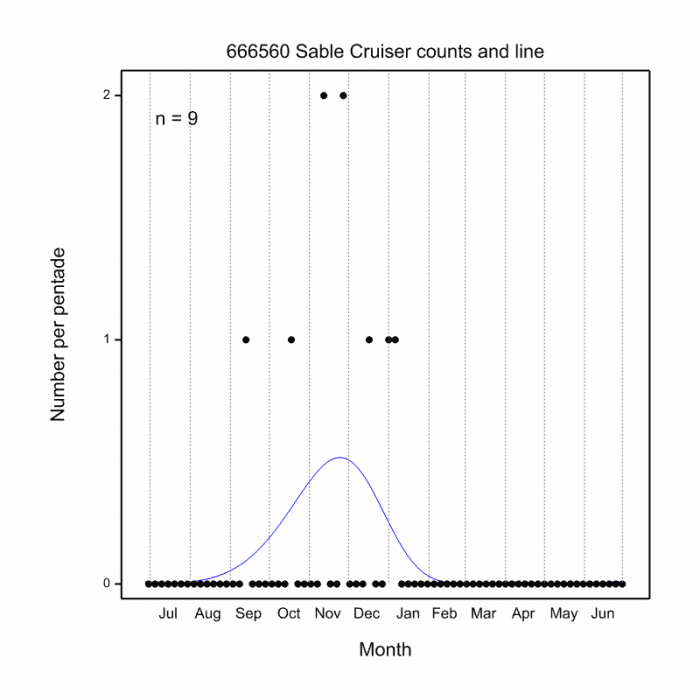
Most active from September to January (see Phenology below).
Status and Conservation
Rare and localised. Listed as Vulnerable in the IUCN Red List of Threatened Species.
Distribution
Phyllomacromia monoceros has a disjunct distribution and occurs in Eastern and Southern Africa. It is found from Kenya in the North down to the Northern Parts of South Africa.
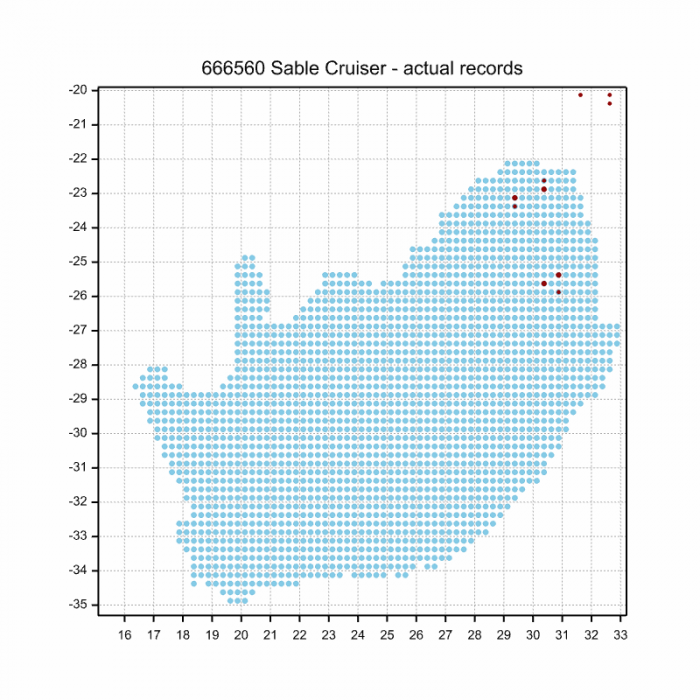
In South Africa the Sable Cruiser has been recorded at several scattered localities along the escarpment in the Limpopo and Mpumalanga provinces.
Below is a map showing the distribution of records for Sable Cruiser in the OdonataMAP database as at February 2020.
The next map below is an imputed map, produced by an interpolation algorithm, which attempts to generate a full distribution map from the partial information in the map above. This map will be improved by the submission of records to the OdonataMAP section of the Virtual Museum.
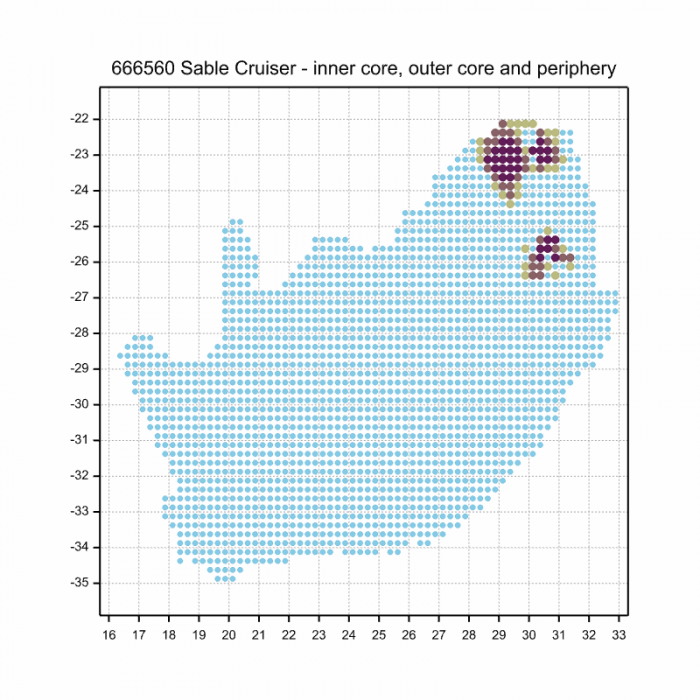
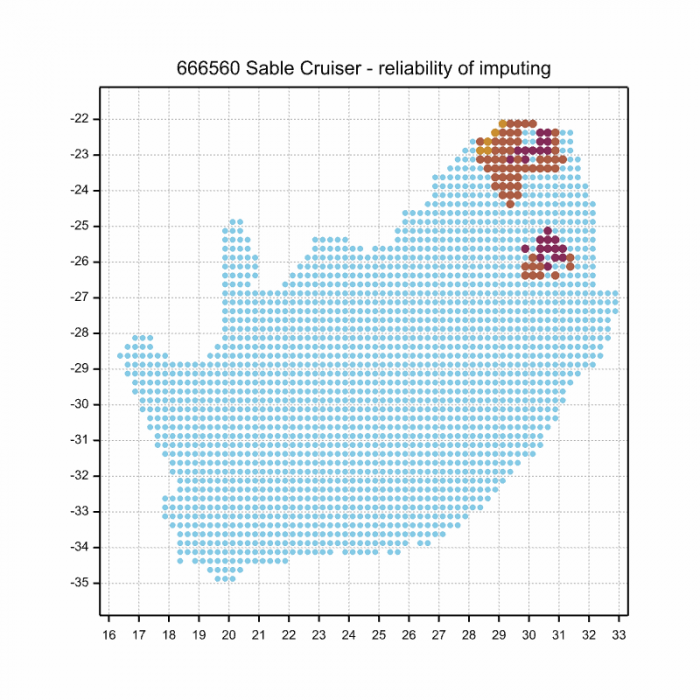
Ultimately, we will produce a series of maps for all the odonata species in the region. The current algorithm is a new algorithm. The objective is mainly to produce “smoothed” maps that could go into a field guide for odonata. This basic version of the algorithm (as mapped above) does not make use of “explanatory variables” (e.g. altitude, terrain roughness, presence of freshwater — we will be producing maps that take these variables into account soon). Currently, it only makes use of the OdonataMAP records for the species being mapped, as well as all the other records of all other species. The basic maps are “optimistic” and will generally show ranges to be larger than what they probably are.
These maps use the data in the OdonataMAP section of the Virtual Museum, and also the database assembled by the previous JRS funded project, which was led by Professor Michael Samways and Dr KD Dijkstra.
Phenology